Proteins known as enzymes serve as biological catalysts by quickening chemical processes. Substrates are the molecules that enzymes can interact with, and the enzyme changes the substrates into other molecules known as products. Enzyme catalysis is required for the majority of metabolic processes in the cell to proceed at speeds quick enough to support life. Enzymes are required for each step in the catalysis of metabolic pathways. Enzymology, the study of enzymes, and the pseudoenzyme analysis area acknowledge that some enzymes have lost the capacity to perform biological catalysis during evolution, which is frequently reflected in their amino acid sequences and strange “pseudocatalytic” capabilities.
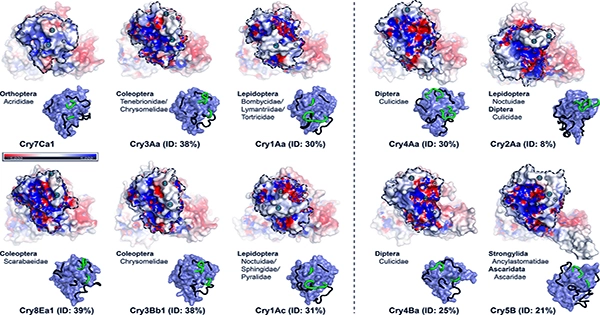
It is known that enzymes catalyze more than 5,000 different kinds of biological reactions. Ribozymes, which are catalytic RNA molecules, are another type of biocatalyst. The specificity of enzymes is derived from their distinctive three-dimensional architectures.
Enzymes, like all catalysts, speed up reactions by reducing their activation energy. Some enzymes have the ability to speed up substrate-to-product conversion millions of times. Orotidine 5′-phosphate decarboxylase is an extreme example, as it speeds up a reaction that would typically take millions of years to complete to milliseconds. Enzymes are similar to any catalyst chemically and neither consume substances in chemical processes nor change the equilibrium of a reaction. Enzymes are far more specialized than the majority of other catalysts. Other compounds, such as activators and inhibitors, can influence the activity of enzymes. Activators reduce enzyme activity, while inhibitors increase it. Enzyme inhibitors are found in many medicinal substances and toxins. Outside of its ideal temperature and pH range, an enzyme’s activity drops significantly, and many enzymes become (permanently) denatured when exposed to high temperatures, losing their structure and catalytic capabilities.
Some enzymes are employed in the production of goods, such as antibiotics. Biological washing powders, which break down protein, starch, or fat stains on clothing, and meat tenderizers, which break down proteins into smaller molecules to make the meat easier to chew, are just a few examples of household items that use enzymes to speed up chemical reactions.