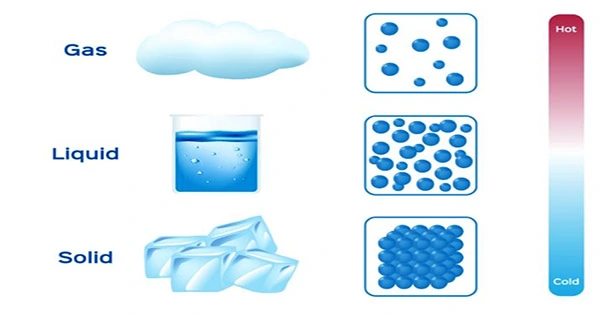
Anything with mass and volume is considered matter (takes up space). It is pretty straightforward to show that the majority of everyday items have mass and occupy space. However, you might be able to picture how challenging it was for individuals in antiquity to provide evidence that air had mass and volume. When compared to an equal amount of solids and liquids, the masses of air (and all other gases) are incredibly small, making them relatively easy to compress (change volume). It would have been challenging to persuade people that gases are matter without delicate apparatus. To find the additional mass brought on by the air within, we can now weigh a little balloon while it is deflated, blow it up, tie it off, and then weigh it again. A one-quart jar holds roughly 0.0002 pounds of air when it is at ambient temperature. Before balances were made to precisely measure very small masses, it would have been challenging to measure this tiny quantity of mass. Gases were later compressed into such a small volume by scientists that the gases transformed into liquids, demonstrating that gases are matter.
The stuff that makes up all of these “things” is made up of a very small number of building blocks, despite the fact that the cosmos contains “things” that are as drastically different as ants and galaxies. Scientists have so far identified or manufactured 118 distinct sorts of these building blocks, which are known as atoms. Each unique sort of atom has a name that was given by scientists. An element is a material that contains only one kind of atom. The fact that our universe’s various kinds of matter are created using just 118 distinct building components should astound you at this point.
In some ways, it’s similar to preparing a five-course, gourmet meal with just three components! What makes it possible? You must comprehend how various elements combine to create matter in order to respond to that question. The creation of molecules is the primary mechanism by which nature arranges atoms into matter. Molecules are collections of two or more atoms that have formed a bond. There are countless methods to join atoms together, which leads to an infinite number of potential molecules.
Chemists are primarily interested in the chemical characteristics that each of these molecules have because they are unique. In a later section of the textbook, you will find a lot more information about atoms and molecules, including how they were found.